The february 2018 revised model clinical trial agreement mcta and clinical research organisation model clinical trial agreement cro mcta templates are designed to be used without modification for industry sponsored trials in nhshsc patients in hospitals throughout the uk health service. Sample clinical trial agreement.
Confidentiality and nda template.
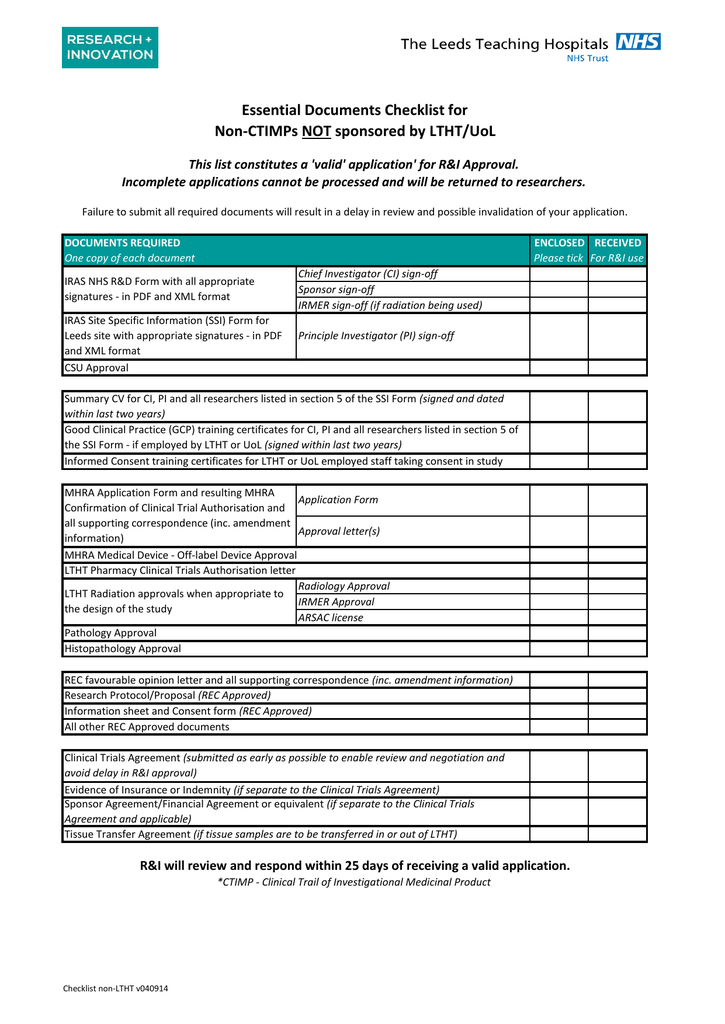
Clinical trial site agreement template. Clinical trial research agreement medicines australia standard form 147k. The pennsylvania state university and. The purpose of this agreement is to lay down the terms of the clinical trial.
Template for clinical trial agreements developed by jim snipes covington burling llp this template and a companion material transfer agreement template were developed by james snipes of covington burling llp in the course of conducting a research paper commissioned by the iom forum on drug discovery development and translation the. And this clinical trial agreement hereinafter referred to as agreement is entered into by and between the pennsylvania state university if applicable and the milton s. Serious adverse events form template.
Clinical trial agreement cta with sponsors or contract research organisations cros sop. Save sign print and download your document when you are done. It is important to protect the interests of both partiesdownload pdf.
Clinical trial agreement log. From these discussions it was also evident that an additional agreement was required for phase 4 clinical trials where a contract research organisation is acting as the local sponsor. In this post ill share nine essential components of a clinical trial agreement cta.
Accelerated clinical trial agreement acta the accelerated clinical trial agreement acta was prepared with the intent to facilitate relationships with industry sponsors that are interested in expediting the contract process thus optimizing lag time for research. Interactions with iec institutional ethics committee sop. A clinical trial agreement is an agreement between an institution and a sponsor of a clinical trial as to how the clinical trial shall be conducted.
Youll learn the purpose of these components and how they can protect you in the event of a conflict or a disagreement. Hershey medical center located at 500 university drive hershey pa 17033 located at. Or a clinical site ended up publishing data from trial subjects without giving the sponsor an opportunity to review the results.
More than just a template our step by step interview process makes it easy to create a clinical trial agreement. The revised templates along with the new phase 4 cro template are available below. Agreements approvals and contracts.
All deliverables conducted by sponsor shall remain property of sponsor both during and upon completion of trial. This section of the clinical trial agreement template provides you with an area to document all ownership rights between parties as well as any other rights to property involved in the clinical trial agreement.








0 Response to "Clinical Trial Site Agreement Template"
Post a Comment